

A revolutionary depression treatment that has changed the field of mental healthcare, Deep TMS™ has been FDA-cleared to safely and effectively treat Major Depressive Disorder (MDD) through BrainsWay’s patented H-coil technology.
Deep TMS treatment for depression works by utilizing a magnetic field that manages to directly reach wider and deeper brain regions, regulating the neural activity of brain structures related to major depressive disorder – specifically the dorsolateral prefrontal cortex (DLPFC).
Repeated peer-reviewed studies have found Deep TMS to be a highly effective treatment that can facilitate a profound improvement in patients’ mental health, alleviating symptoms of depression and contributing to a significantly greater quality of life.
As a noninvasive procedure, Deep TMS is a well-tolerated treatment that does not cause any adverse or long-lasting side effects. It does not require a significant recovery period, and the 20-min treatment can easily be integrated into each patient’s day-to-day schedule.
 Depression, or major depressive disorder (MDD), is defined by the American Psychiatric Association as a mood disorder characterized by a significant decrease in one’s quality of life across several different fields.
Depression, or major depressive disorder (MDD), is defined by the American Psychiatric Association as a mood disorder characterized by a significant decrease in one’s quality of life across several different fields.
Emotionally, depression normally involves a great deal of sadness, loneliness, and a lack of hope or pleasure. It also brings about detrimental thoughts and actions that decrease the individual’s level of functioning.
Read More about the history of depression
Depression Demographics: According to the National Institute of Mental Health, Depression affects roughly one in 15 adults (6.7%). In the US, 17.3 million adults (7.1%) report experiencing one or more depressive episodes across their lifetime.
Possible Causes for Depression: Several risk factors have been found to increase the chance of developing MDD. These include genetics, temperamental inclination, childhood environment, significant life events and the existence of a medical condition or another psychiatric disorder.
 Thanks to major breakthroughs in research and fieldwork, a number of mental health treatments have been developed to safely and effectively treat MDD. Among them is Deep TMS, which was developed to address unmet needs that arose from legacy MDD treatments:
Thanks to major breakthroughs in research and fieldwork, a number of mental health treatments have been developed to safely and effectively treat MDD. Among them is Deep TMS, which was developed to address unmet needs that arose from legacy MDD treatments:
Medical Device Treatments: A number of cutting-edge medical technologies treat depression, with ECT, TMS, and Deep TMS gaining professional recognition.
ECT: Electroconvulsive therapy induces a brief set of seizures that stimulate the brain’s neural activity. Originally used to treat schizophrenia, ECT was eventually found to help alleviate severe depression, and today is primarily used to treat this condition.
Though highly effective, ECT is not for everyone. It requires full sedation under anesthesia, which complicates the recovery process. Its side effects can be severe and include short-term memory loss. And due to a spread of misinformation surrounding this treatment, many patients are wary of trying it out.
Traditional rTMS: Available to the public since 2008, Transcranial Magnetic Stimulation is a noninvasive treatment for depression. The procedure sends out electromagnetic pulses from a figure-8-shaped handheld device, used to regulate the neural activity of brain structures found to be involved in depression.
While rTMS has been clinically shown to be a safe and effective treatment option, this original, traditional form of TMS has certain limitations: The figure-8 coil’s relatively narrow scope means only a focal brain structure can be regulated at any given moment, causing targeting issues to arise during treatment. Standard rTMS also has trouble stimulating deeper structures directly, which can further affect its efficacy.
Deep TMS: Deep Transcranial Magnetic Stimulation is an advancement on the standard, figure-8 rTMS treatment that manages to address the concerns raised with its predecessor. Deep TMS is also a noninvasive treatment that utilizes a magnetic field to safely and effectively regulate the neural activity of brain structures associated with depression, in addition to other forms of mental illness. Deep TMS does not require anesthesia or recovery, and can therefore be incorporated into an individual’s daily routine.
Deep TMS’s high levels of safety and effectiveness granted it an FDA clearance status in 2013 for treating depression. Deep TMS is also the only noninvasive medical device to be given an FDA clearance based on peer-reviewed clinical data for treating OCD and Smoking Addiction. It is additionally CE-marked in Europe, to treat depression, OCD and several other mental health conditions.
 Psychotherapy: Psychotherapy, or talk therapy, refers to speaking with a mental health professional to better understand one’s experiences and increase their wellbeing. Forms of psychotherapy commonly used to treat depression include psychodynamics, cognitive-behavioral therapy (CBT) and interpersonal therapy (IPT). Support groups for patients and their primary care providers have also been shown to offer relief.
Psychotherapy: Psychotherapy, or talk therapy, refers to speaking with a mental health professional to better understand one’s experiences and increase their wellbeing. Forms of psychotherapy commonly used to treat depression include psychodynamics, cognitive-behavioral therapy (CBT) and interpersonal therapy (IPT). Support groups for patients and their primary care providers have also been shown to offer relief.
Psychopharmacology: Several classes of medication have been shown to successfully treat depression. They include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs). Though psychopharmacology is a generally effective MDD treatment, it can cause side effects such as weight gain or sexual dysfunction, and many patients are found to be treatment-resistant.
Lifestyle Changes to Protect Against MDD: While the above-mentioned factors are more stable fixtures, choosing to implement certain lifestyle changes can also help ward off depression. These include: adopting a Mediterranean diet rich with fruit, vegetables, olive oil and fish; participating in exercise to help release mood-elevating endorphins, strengthen self-worth and distract from depressive triggers; replenishing your energy levels through a good night’s sleep; and developing a balanced work-rest-play dynamic.
Deep TMS has repeatedly been shown to safely and effectively alleviate depressive symptoms. Its high efficacy level is due to the magnetic field produced by Deep TMS’s H-Coil, able to reach wider areas of the brain while also directly stimulating those structures located in its deeper regions.
An independent study* published in 2019 by the Journal of Psychiatric Research determined that Deep TMS plus standard medication was significantly more effective at reducing depression levels among MDD patients compared with traditional rTMS plus medication or medication alone.
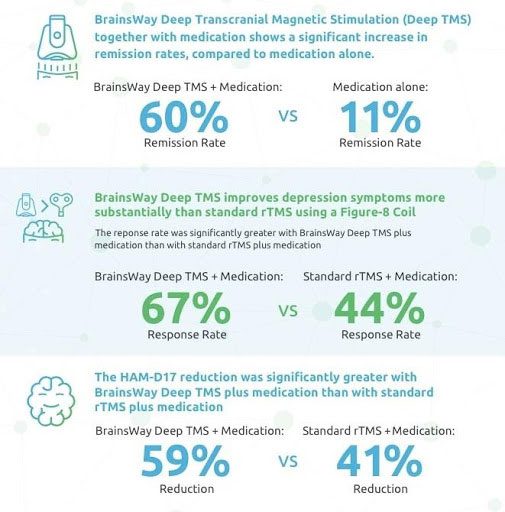
*The Magstim® Rapid² device was used in this study. (Magstim Company, Spring Gardens, UK)
In 2015, a multicenter, sham-controlled study in World Psychiatry confirmed its efficacy independent of medication effects, finding that one out of three patients with treatment-resistant MDD achieved remission with Deep TMS during the treatment’s acute phase.
The study also found that nearly 80% of patients who did not immediately respond during the acute treatment phase, proceeded to experience an improvement during the continuation phase.
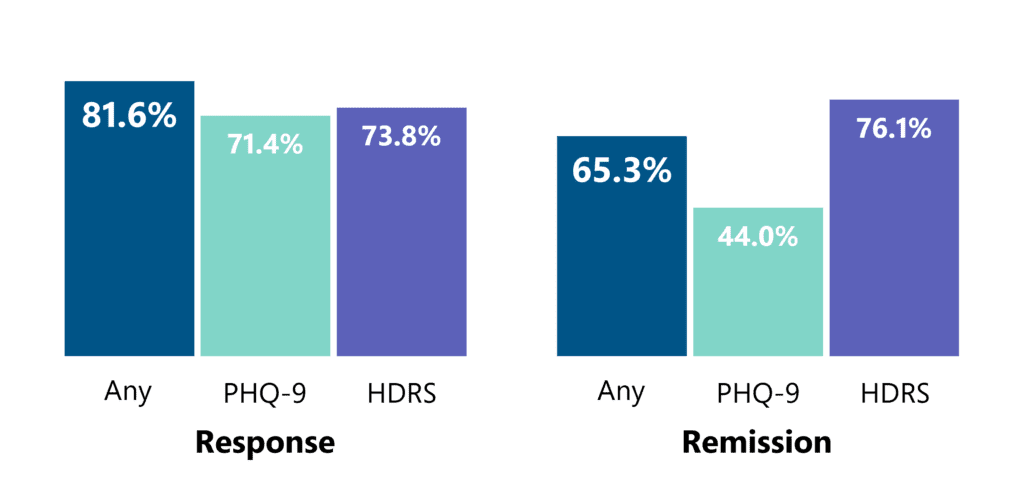
Based on recorded data of over 1,300 patients, Deep TMS has been shown to have an even greater effect in a real-life clinical practice setting. Among patients who completed at least 30 sessions, approximately 4 out of 5 achieved a clinical response and approximately 2 out of 3 achieved remission from depression.
 Deep TMS has not been shown to cause any substantial or lasting side effects. A 2007 study in Clinical Neurophysiology confirmed this, finding Deep TMS to be a well-tolerated treatment that does not cause any adverse side effects.
Deep TMS has not been shown to cause any substantial or lasting side effects. A 2007 study in Clinical Neurophysiology confirmed this, finding Deep TMS to be a well-tolerated treatment that does not cause any adverse side effects.
Individuals undergoing Deep TMS treatment may experience a slight discomfort during the procedure, with some reporting a tapping sensation in the area being treated. The most common side effects are as follows:
Side effects are typically reported to have passed shortly after initial treatment sessions.
BrainsWay’s unique, patented H-Coil technology is held inside a cushioned helmet fitted onto the patient’s head. Upon activation, the H-coil produces magnetic pulses that regulate the brain’s neural activity.
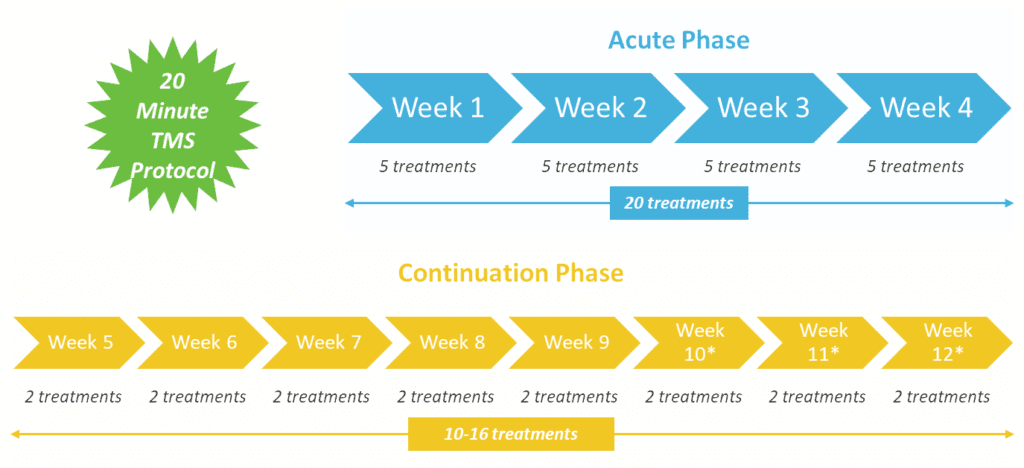
BrainsWay’s Deep TMS sessions for depression last approximately 20 minutes, as opposed to 40-minute standard rTMS sessions. Alternatively, a 3-min intermittent theta burst (iTBS) session is also available. A Deep TMS treatment plan usually requires daily sessions over four weeks, followed by periodic maintenance sessions.
The following graphic details Deep TMS’s treatment protocol, with its Acute and Continuation phases:
Hear how a depression patient developed a new outlook on life after BrainsWay Deep TMS depression treatment with Dr. John Zebrun of Achieve TMS East.
Check out additional testimonials of patients and healthcare providers in our video gallery.
A unique and innovative treatment, called Deep TMS, is now poised to offer true relief to people who...
It is estimated that over 15 million Americans suffer from Major Depressive Disorder (MDD). MDD is a...
BrainsWay Introduces the First FDA Cleared (De-Novo), Non-invasive, Medical Device for the Treatment...
Anti-psychotic medications are often effective for the positive symptoms of schizophrenia
Post-traumatic stress disorder (PTSD) is an extreme anxiety response to a life-threatening situation