BrainsWay’s Deep Transcranial Magnetic Stimulation (Deep TMS™) is a unique, non-invasive treatment that utilizes magnetic fields to treat various mental health conditions, by effectively regulating the neural activity of brain structures found to be associated with those conditions. FDA-cleared to treat both depression and OCD, Deep TMS has been shown to be well-tolerated and proven effective in alleviating adverse symptoms.
During the Deep TMS treatment process, a padded helmet containing BrainsWay’s patented H-coil is fitted over the patient’s head. The helmet then sends out magnetic pulses that influence the neural activity of the relevant brain structures. The non-invasive treatment process does not require anesthesia and each session lasts about 20 minutes, allowing you to incorporate them into your daily routine.
With Deep TMS technology at the forefront of mental health therapy, BrainsWay continues to strive to offer relief to patients in need.
A growing body of research has found Deep TMS to successfully alleviate symptoms of mental health conditions. These studies use gold-standard benchmarks, such as randomized, double-blind protocols and in-depth analyses, to reach solid, evidence-based conclusions. They have been published in such well-regarded journals as The American Journal of Psychiatry, World Psychiatry and the Journal of Psychiatric Research.
Due to its success in offering symptom relief and its favorable safety profile, Deep TMS has already been granted FDA clearance to treat both major depressive disorder (MDD) and obsessive-compulsive disorder (OCD).
A 2015 study published in World Psychiatry highlighted Deep TMS’s effect on treatment-resistant participants with MDD. The study, aggregating data from 20 academic medical centers, concluded that Deep TMS treatment led to remission in roughly one in three participants following the acute phase’s four weeks of treatment. Study participants were treatment-resistant patients who had previously not significantly benefited from antidepressant medication. Results from this study were considered so conclusive as to earn Deep TMS its first FDA clearance status.
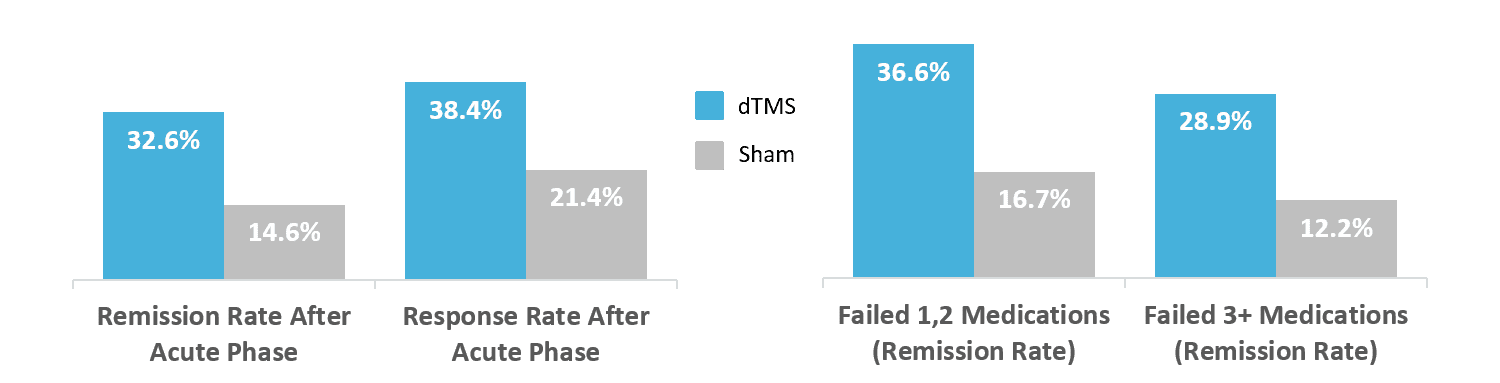
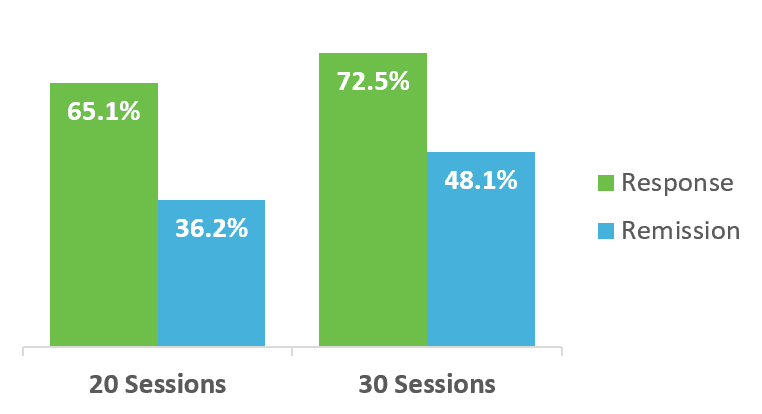
Deep TMS has been shown to be even more effective in real-life clinical settings —often as an adjunct form of therapy together with other treatments, such as antidepressant medication and psychotherapy. Based on the recorded data of over 1000 participants undergoing Deep TMS treatment for MDD, 75% achieved a clinical response, with one in two achieving remission.
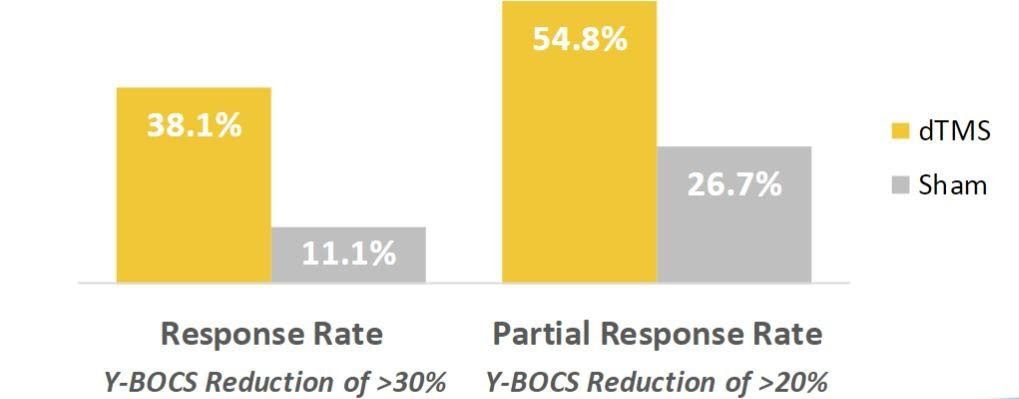
In addition to depression, Deep TMS has also been FDA-cleared (De Novo) to treat obsessive-compulsive disorder (OCD). Its efficacy in treating this condition was demonstrated in a 2019 publication in the American Journal of Psychiatry. The study utilized the Y-BOCS rating scale, considered the gold standard tool used by psychologists and psychiatrists to measure the severity of OCD.
A “response rate” was defined as a reduction of at least 30% of the subjects’ symptoms after undergoing Deep TMS therapy, and a “partial response rate” was defined as a reduction of at least 20%. The study found that Deep TMS treatment offers significant clinical improvement for treatment-resistant patients, with more than half of patients achieving a partial response, and about a third of patients achieving a response.
The study demonstrated efficacy even among patients who had previously been found unresponsive to either psychotherapy or medication—which make up a large percentage of those battling OCD. Furthermore, these patients’ improvement was sustained for several weeks following the treatment’s conclusion.
Deep TMS is a well-tolerated treatment option with a favorable safety profile. The Deep TMS system is operated by trained professionals required to follow specific guidelines that are designed to ensure the patient’s safety. It is a non-invasive treatment course that is not associated with any systemic side effects. Extra care is provided to facilitate a comfortable experience for the patient.
A 2019 study published by the Journal of Psychiatric Research underscored Deep TMS’s advantages, by finding that a combination of Deep TMS with antidepressant medication was a safe treatment course that offered significant greater symptom relief than antidepressant medication alone. These results show that Deep TMS has the ability to enhance existing therapies, while offering its own unique advantage when treating issues of mental health.
Individuals undergoing Deep TMS treatment may experience a mild headache or scalp discomfort during the procedure itself, with some reporting a tapping sensation in the targeted area. Generally, side effects such as headaches that may arise following the Deep TMS procedure are mild, and tend to pass within a short amount of time. There is also a very rare risk of seizure associated with the treatment.
Side effects prevalent with other treatments like weight gain, sexual dysfunction or memory effects were not observed in patients following Deep TMS treatment.
All individuals considering Deep TMS are encouraged to discuss this treatment with their doctor, to determine whether this treatment is right for them.
Out of concern for their personal safety, individuals included in the following categories should not undergo Deep TMS treatment:
Additionally, the safety and efficacy of BrainsWay’s Deep TMS have not specifically been established in the following patient populations through a controlled clinical trial:
As a result of medical breakthroughs in the mental health field and an increased demand for innovative treatments, many insurance providers now offer coverage for Deep TMS. A safe, effective, non-invasive and FDA-cleared approach, Deep TMS is being seen as part of a new wave of therapies for medication-resistant patients searching for a path that works for them.
Deep TMS treatment is covered differently by insurance providers around the world. In the United States, where Deep TMS has been FDA-cleared since 2013, coverage is widely available for the treatment of MDD. We estimate that over 90% of the total private insurer adult covered lives in the United States have coverage for MDD treatment with Deep TMS. In addition, Deep TMS for MDD treatment is generally eligible for reimbursement from Medicare.
Deep TMS for OCD may currently be eligible for reimbursement on a single case agreement basis. However, we are working to broaden the scope of reimbursement coverage for Deep TMS to include OCD treatment, based on the unmet clinical need and the efficacy and safety profile of the treatment.
Where covered by insurance, treatment will generally require prior authorization and patients may incur a co-pay per treatment session, as specified by their insurance plan. Patients whose plan does not stipulate Deep TMS coverage can nevertheless speak with their mental health provider, as in some cases single-case opportunities can be made available to cover treatment costs.
BrainsWay partners with outreach organizations such as the National Alliance on Mental Illness (NAMI), Mental Health America, and the International OCD Foundation (IOCDF) to raise awareness for mental health issues among local communities. These partnerships include sponsoring exhibits and educational symposia at conferences, as well as sponsoring local awareness events and fundraisers (e.g. NAMI Walks).
As more individuals educate themselves on ways to cope with and work through different mental health conditions, they also learn about how Deep TMS can help alleviate the debilitating symptoms they are experiencing. This is particularly true for patients with MDD and OCD who are often found to be treatment-resistant to first-line treatments, such as psychotherapy and medication.
In addition to joining forces with healthcare organizations, BrainsWay works closely with Deep TMS healthcare providers to raise awareness among patients and caregivers for Deep TMS as a game-changing treatment option.
These efforts include digital marketing, aimed at increasing online Deep TMS awareness and understanding, as well as highlighting personal patient stories through traditional media, with many local and national news featuring articles on the benefits of Deep TMS. One such outlet was CBS This Morning, which focused on the symptom relief one patient battling depression experienced from Deep TMS treatment. This segment managed to reach millions of viewers, educating many individuals who may be facing this condition’s adverse effects.
BrainsWay also often partners with providers and patients on live events, designed to promote public awareness on the treatment benefits of Deep TMS to members of local communities. These include initiatives like “After the Smoke Clears,” a mental health event sponsored by BrainsWay and Calabasas Behavioral Health in Southern California, which was designed to help members of Los Angeles County cope with a bout of destructive wildfires and a tragic mass shooting.
As part of BrainsWay’s mission to expand the public’s general awareness of mental health-related issues, we have set up a Knowledge Center that addresses the many concerns, treatments and information that is included in this very broad topic.
You can gain a deeper understanding of the scientific breakthroughs that benefit patients of Deep TMS by accessing our Knowledge Center. It includes a comprehensive list of clinical research performed on Deep TMS, numerous articles on mental health education, and information on active trials that you can join today.
In addition, you can listen to patients and healthcare providers share their experience with BrainsWay Deep TMS in the video gallery, and download patient brochures highlighting how BrainsWay can improve your quality of life.