

BrainsWay Deep Transcranial Magnetic Stimulation (Deep TMS™) is a safe and effective aid for short-term smoking cessation in adults, representing the first FDA clearance in the addiction space for any TMS device.
Using BrainsWay’s patented H4 Coil, the treatment generates electromagnetic pulses that stimulate neurons in brain structures associated with addiction – specifically the bilateral insula and prefrontal cortex. BrainsWay Deep TMS is proven to reach wider and deeper brain regions than traditional TMS devices.
A double-blind, sham-controlled, multicenter randomized controlled trial of 262 patients found Deep TMS to be an effective treatment, significantly improving the continuous quit rate, reducing craving and the average number of cigarettes smoked per week. Participants in the study were highly addicted to smoking, with a history of smoking an average of over 26 years and multiple failed attempts to quit.
Deep TMS is a well-tolerated, noninvasive treatment with no systemic side effects. It does not require a significant recovery period, and the 18-min treatment can easily be integrated into each patient’s day-to-day schedule.

Tobacco smoking is one of the leading causes of preventable disease and death throughout the world, with 34.2 million U.S. adults smoking cigarettes and approximately 480,000 dying each year (about 1 in 5 deaths). While the percentage of Americans who smoke cigarettes has declined over the past several decades in part thanks to various impactful public health initiatives, tobacco addiction continues to be a significant health concern, with more than 16 million living with a smoking-related disease, such as lung cancer and heart failure.
Addiction to nicotine, similar to addiction to drugs and alcohol, is characterized by intense craving, compulsive use, and an inability to stop despite negative consequences. Areas of the brain involved in decision-making and behavioral control are impacted, and smokers build a tolerance, requiring increasing levels of nicotine to achieve the same neurotransmitter release that triggers pleasure. In addition to health problems associated with nicotine addiction, smokers can also suffer from withdrawal symptoms when trying to stop and reduced participation in social situations where smoking is not allowed.
Smoking Addiction Demographics: According to the CDC, men are more likely than women to be cigarette smokers (15.6% vs. 12.0%). Smoking is more prevalent among individuals with lower household income and lower degrees of education. Adults experiencing psychological stress have also been shown to smoke at nearly triple the rate of those not experiencing psychological stress.
Possible Cause for Smoking Addiction: Factors that increase the likelihood of nicotine addiction include younger age at which the individual begins smoking, genetic inheritance, individuals who grow up with parents who smoke, and other substance abuse. In addition, numerous studies have demonstrated an association between smoking and mental illness, including depression and PTSD.
Several treatments have been developed to treat smoking addiction, many of which have been found to be effective in combination.
Deep TMS: Deep TMS is the only non-invasive medical device FDA-cleared for short-term smoking cessation. It uses electromagnetic pulses to stimulate neurons in the bilateral insula and prefrontal cortex, reducing tobacco cravings and increasing cognitive control. A double-blind, multicenter, randomized, controlled pivotal study of 262 subjects demonstrated a significant improvement in the continuous quit rate, as well as a substantial reduction in cravings and number of cigarettes smoked. Deep TMS has been shown to be well-tolerated, causing no systemic side effects, and can easily be incorporated into the patient’s daily routine.

Psychopharmacology: Two medications, Bupropion (Zyban) and Varenicline (Chantix), over a 7-12 week course of treatment, have been FDA-approved to treat smoking addiction. Bupropion inhibits the reuptake of norepinephrine and dopamine, thereby increasing their synaptic availability, while Varenicline blocks nicotine receptors. Medication for smoking cessation present potential side effects, including nausea, sleeplessness, and rare seizures, particularly for patients who drink alcohol while taking the drug.
Nicotine Replacement Therapy: A variety of over-the-counter NRTs are available, includes gums, sprays, patches, and lozenges. These treatments provide small amounts of nicotine, helping the quitting smoker cut down on cravings and ease symptoms of withdrawal. NRTs have been shown to be more effective when paired with counseling programs. Possible side effects include skin irritation, dizziness, headache, racing heartbeat, and rarely nicotine overdose.
Psychotherapy: Counseling helps smokers develop the skills, behavioral and mental patterns needed to cease addiction to tobacco. Several forms of psychotherapy have been shown in clinical studies to be effective for smoking addiction. Cognitive Behavioral Therapy (CBT) helps smokers identify triggers and teaches coping strategies when presented with those triggers. Motivational Interviewing (MI) helps smokers understand their reluctance to quit and improves motivation to live a healthy lifestyle. Mindfulness teaches smokers to become aware of cravings and reframe them as tolerable.
Lifestyle Changes to Protect Against Smoking Addiction: In addiction to medical treatments, improvements to diet, exercise, and avoiding smokers have been shown to contribute to cessation. Note that e-cigarettes have not been proven to be safe or effective for smoking cessation, and patients attempting to quit should avoid substituting other forms of tobacco.
BrainsWay’s Deep TMS treatment using the H4 Coil aids in short-term smoking cessation, as demonstrated by data obtained in BrainsWay’s multicenter, double-blind, randomized controlled pivotal study. Participants in the study were highly addicted to smoking, with a history of smoking on average for over 26 years and multiple failed attempts to quit.
The study demonstrated that among participants that completed treatment, the continuous quit rate, referring to four consecutive weeks of abstinence from smoking, was 28.0% in the active Deep TMS treatment group compared to 11.7% in the sham treatment group. Weekly abstinence was self-reported by participants and corroborated through urine tests.
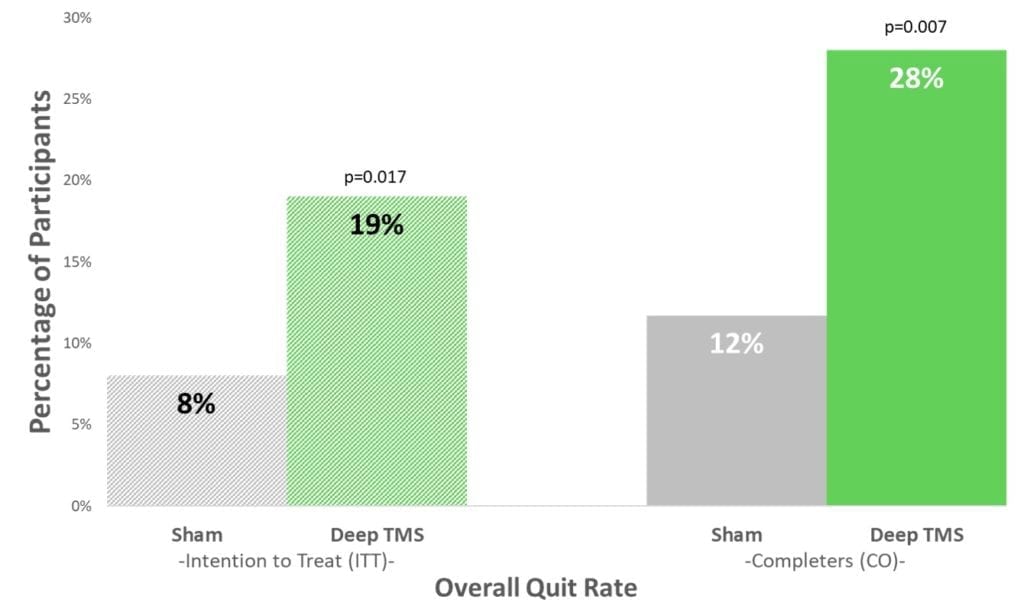
In addition, the difference in the average number of cigarettes smoked per day per subject from baseline to the end of the study (4-month follow-up for quitters and 6 weeks follow-up for smokers) was statistically significantly lower in the active Deep TMS group compared to the sham group.
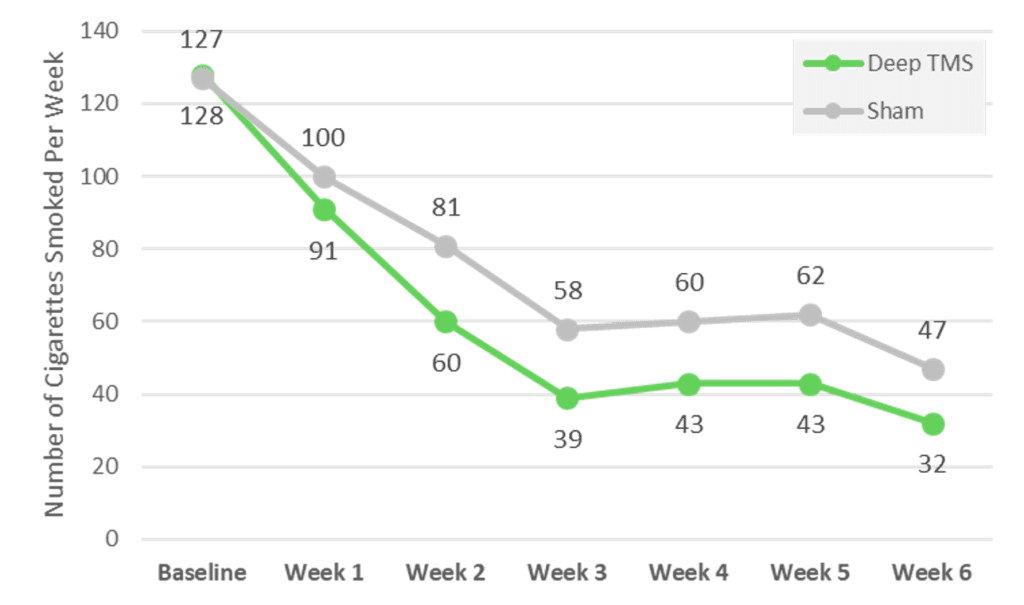
Deep TMS treatment for treatment of smoking addiction is noninvasive, requiring no anesthesia, does not cause any significant systemic side effects, and can easily be incorporated into the patient’s routine.
In the pivotal clinical study, Deep TMS was shown to be well-tolerated. The most common side effect reported was headache, primarily during the first treatment sessions (24% of those in the active treatment group and 18% in the sham group). No seizures were observed.
BrainsWay’s Deep TMS for Smoking Addiction consists of a patented H-Coil, held inside a cushioned helmet fitted onto the patient’s head. When activated, the H-Coil produces repetitive magnetic pulses that regulate the brain’s neural activity.
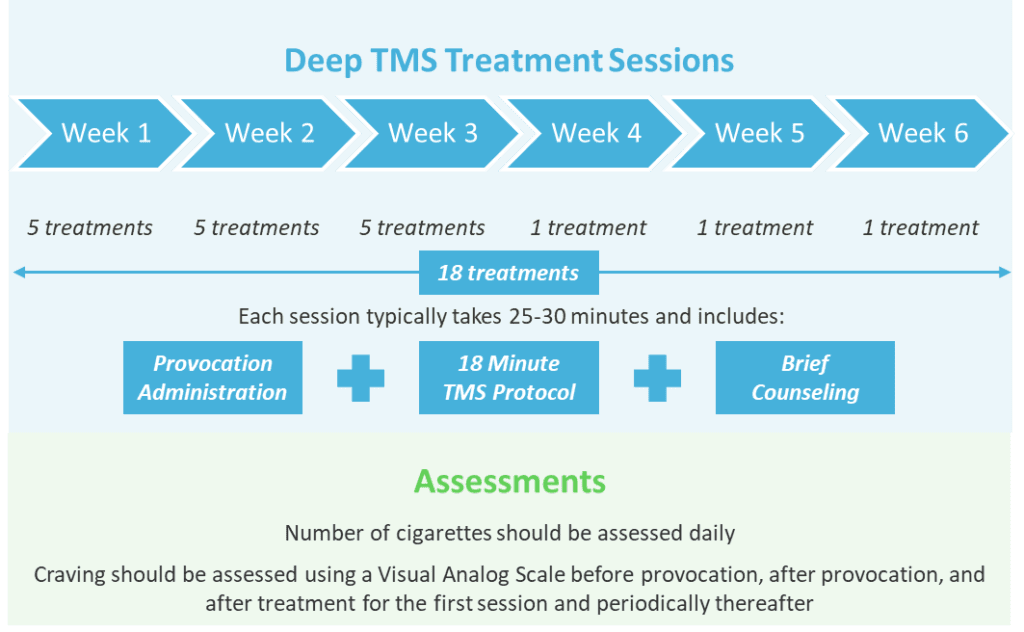
Deep TMS sessions for treatment of smoking addiction consists of a brief provocation designed to activate the relevant brain circuitry, an 18-minute TMS treatment, and brief counseling. A full course of BrainsWay smoking addiction treatment typically requires three weeks of daily treatments, followed by one treatment per week for three additional weeks (18 sessions total over six weeks).
The target quit date should be set within the first 2 weeks of treatment. Many patients stop smoking within the acute phase of treatment, with the remaining treatments helping maintain the effect.
Depression is a neuropsychiatric disorder that is expressed in emotional, physiological, and behavioral...
BrainsWay Introduces the First FDA Cleared (De-Novo), Non-invasive, Medical Device for the Treatment...
Deep TMS is the only TMS system FDA-cleared to reduce comorbid anxiety symptoms in adult patients with...