Journal: World Psychiatry 20:1-8 (2021)
Authors: A Zangen, H Moshe, D Martinez, N Barnea-Ygael, T Vapnik, A Bystritsky, W Duffy, D Toder, L Casuto, M Lipkinsky Grosz, E.V Nunes, H Ward, A Tendler, D Feifel, O Morales, Y Roth, D.V Iosifescu, J Winston, T Wirecki, A Stein, F Deutsch, X Li, M.S George
Background:
Deep TMS is a non-invasive brain stimulation method increasingly used to treat psychiatric disorders, primarily depression. Initial studies suggest that Deep TMS may help to treat addictions, but evaluation in multicenter randomized controlled trials (RCTs) is needed.
Objective:
This multicenter double-blind RCT intended to evaluate the safety and efficacy of Deep TMS using the H4 coil over the bilateral prefrontal and insular cortex as an aid in smoking cessation.
Methods:
The trial included 262 chronic smokers meeting DSM-5 criteria for tobacco use disorder, who had made at least one prior failed attempt to quit, with 68% having made at least three failed attempts. They received three weeks of daily bilateral active or sham Deep TMS to the lateral prefrontal and insular cortices, followed by once weekly Deep TMS for three weeks. Each Deep TMS session was administered following a cue-induced craving procedure, and participants were monitored for a total of six weeks. Those in abstinence were monitored for additional 12 weeks.
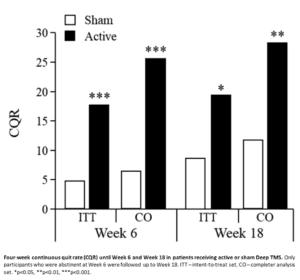
The primary outcome measure was the four-week continuous quit rate (CQR) until Week 18 in the intent-to-treat efficacy set, as determined by daily smoking diaries and verified by urine cotinine measures. The trial was registered at ClinicalTrials.gov (NCT02126124).
Results:
In the intent-to-treat analysis set (N=234), the CQR until Week 18 was 19.4% following active and 8.7% following sham Deep TMS (X2=5.655, p=0.017). Among completers (N=169), the CQR until Week 18 was 28.0% and 11.7%, respectively (X2=7.219, p=0.007). The reduction in cigarette consumption and craving was significantly greater in the active than the sham group as early as two weeks into treatment.
Conclusions:
This study establishes a safe treatment protocol that promotes smoking cessation by stimulating relevant brain circuits. It represents the first large multicenter RCT of brain stimulation in addiction medicine and has led to the first clearance by the US Food and Drug Administration for Deep TMS as an aid in smoking cessation for adults.