Journal: Journal of Psychiatric Research (2019)
Authors: I Filipčić, I šimunović Filipčić, Ž Milovac, S Sučić, T Gajšak, E Ivezić, S Bašić, Ž Bajić, M Heilig
Repetitive transcranial magnetic stimulation (rTMS) is an evidence-based treatment option for major depressive disorder (MDD). However, comparisons of efficacy between the two FDA-approved protocols of rTMS modalities are lacking.
The aim of this industry-independent, randomized-controlled, single-blind trial was to evaluate clinical outcome of the two FDA-approved rTMS protocols delivered by H1-coil and the figure-8-coil*, in MDD patients.
*The Magstim® Rapid² device was used in this study. (Magstim Company, Spring Gardens, UK)
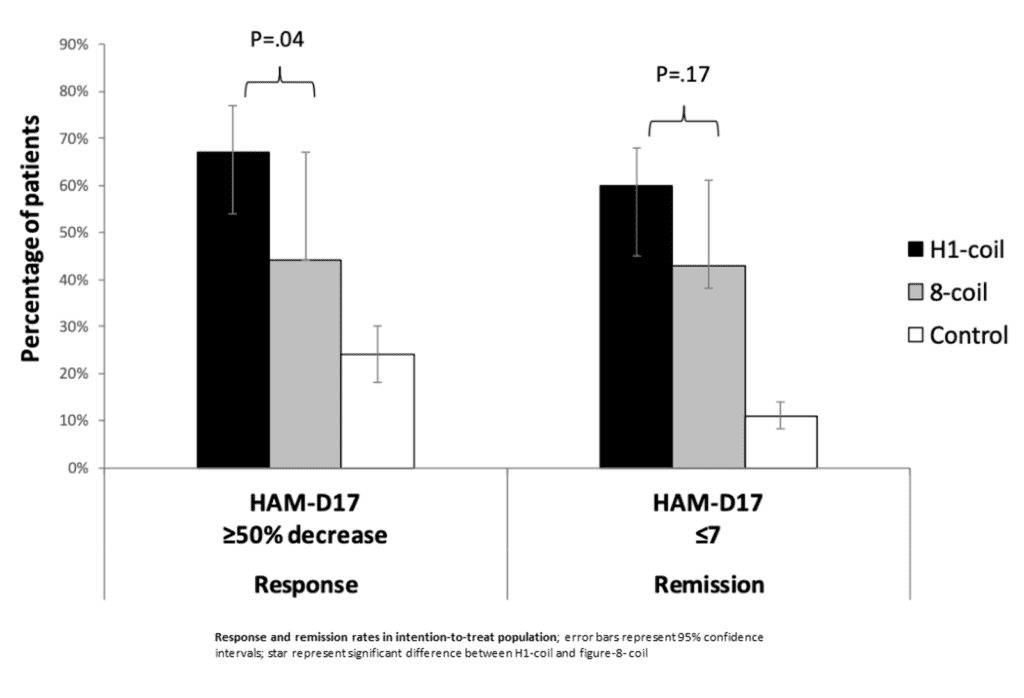
A total of 228 MDD patients were randomized to 20 sessions of H1-coil or 8-coil as an adjunct to standard-of-care pharmacotherapy, or standard-of-care pharmacotherapy alone. Baseline MDD symptom severity was almost the same in the three groups. Hamilton depression rating scale (HAM-D17) mean score was 17 (5.3) in H1-coil, 17 (5.4) in 8-coil, and 19 (6.1) in control group. The primary outcome was the proportion of patients achieving remission defined as HAM-D17 score ≤7 at end-of-treatment at week-4.
In the intention-to-treat analysis odds ratio for remission was 1.74 (CI95% 0.79–3.83) in H1-coil compared to the 8-coil group. The difference between two rTMS protocols was not significant. Remission rate was significantly greater in both HF-rTMS groups compared to the control: 60% (CI95% 48–71%), 43% (CI95% 31–55%) and 11% (CI95% 5–20%) respectively. The response was significantly better in H1-coil, than in 8-coil group OR = 2.33; CI95% 1.04–5.21 (P = 0.040). The HAM-D17 was lowered by 59% in the H1-coil, 41% in the 8-coil (P = 0.048), and 17% in the control group (P < 0.001 vs H1-coil; P = 0.003 vs 8-coil). Safety, tolerability, and the changes in quality of life were comparable.
The authors confirmed the safety and efficacy of both FDA-approved protocols as adjunctive treatments of MDD. Better response rate and greater reduction of depression severity were observed in the H1-coil group, but without a significant difference in the remission rate between the two rTMS modalities.