This Patient Manual is a supplement to the BrainsWay Deep TMSTM System Instructions for Use.
If you are considering BrainsWay Deep TMS treatment for treatment of OCD you should review this patient manual and discuss the information with your doctor in order to understand more about:
CAUTION: Federal law restricts this device to sale by or on the order of a licensed clinician.
You have been diagnosed with Obsessive-Compulsive Disorder (OCD) and your doctor has recommended treatment with the BrainsWay Deep TMS System to treat symptoms of OCD. BrainsWay Deep TMS treatment has been shown to be safe and effective in the treatment of patients with OCD.
Your Healthcare Provider (psychiatrist or physician) will review your medical and OCD history to help determine if BrainsWay Deep TMS treatment is appropriate for you or not.
The treatment is performed under your healthcare provider’s supervision. Neither anesthesia nor sedation is required. You will be awake and alert during the treatment sessions. The treatment sessions include daily treatments for a period of 6 weeks. Each treatment session lasts approximately 20-30 minutes.
Deep TMS stands for “Deep Transcranial Magnetic Stimulation”. Deep TMS is a noninvasive procedure, involving stimulation of a part of the brain called the Anterior Cingulate Cortex. The stimulation activates nerve cells in this region of the brain and improves OCD symptoms.
The stimulation is produced by a treatment “coil” contained in a helmet. During treatment, the coil is gently positioned on the frontal side of your head, over this region of the brain. Short pulses of electricity sent through the treatment coil generate magnetic fields that turn on and off very rapidly. These magnetic fields are similar to those used in magnetic resonance imaging (MRI) systems. The magnetic field goes through the head and induces a weak electrical current that briefly activates the nerve cells in this region of the brain and improves OCD symptoms.
Patients already on OCD treatments should be maintained at their current dosages during the Deep TMS treatment.
The BrainsWay Deep TMSTM System is intended to be used as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder.
Deep TMS treatment delivers a magnetic field that could cause any metal objects that are near the device to move or to get hot. Deep TMS treatment should not be used if you have metal implants in or around your head (except for standard amalgam dental fillings). Deep TMS treatment should not be used if you have implanted electronic devices in your head. These implants could cause serious injury or death if Deep TMS treatment is used. You should tell your healthcare provider if you have any metal devices or objects in your head or body in order to determine if those devices could be affected by the treatment.
Safety of the BrainsWay Deep TMS System was demonstrated in a clinical study involving 100 patients with moderate to severe Obsessive-Compulsive Disorder. The patients ranged in age from 22 to 68 years.
This section summarizes the adverse events and side effects reported in the clinical study with the BrainsWay Deep TMS System.
You should discuss the warnings and precautions related to the Deep TMS treatment with your healthcare provider to determine if any precautions should be taken prior to or during your treatment with the BrainsWay Deep TMS System.
There were no cases of a seizure (also called convulsions) reported in the clinical study with the BrainsWay Deep TMS System. In previous studies with the BrainsWay Deep TMS System, 7 seizures (out of over 300,000 treatment sessions) have been reported in subjects on high doses of psychotropic medications, which are known to increase the risk of a seizure, due to high alcohol consumption the night before treatment, or due to other reasons. None of the subjects who have experienced Deep TMS induced seizures have suffered lasting physical sequelae.
You should discuss with your doctor if you have had a seizure, or if you have a medical condition or change in a medical condition which may put you at increased risk of having a seizure, e.g., brain injury, change in medications, change in electrolyte balance, etc. Your doctor will decide if it is appropriate for you to receive BrainsWay Deep TMS treatment.
BrainsWay Deep TMS treatment may require several weeks of treatment before symptom improvement occurs.
You should also inform your doctor if you have any behavioral changes, such as worsening of OCD symptoms, suicide attempts and ideations, increased aggressiveness, euphoria, or irritability. Your family and caregiver should also be aware that if such behavioral changes appear, they should inform the treatment administrator immediately. Your doctor will determine whether BrainsWay Deep TMS treatment should be discontinued and, if so, what other treatment options are available.
The long-term safety of the BrainsWay Deep TMS treatment has been demonstrated in a clinical study. During the full course of 6 weeks of ongoing treatment, the treatment was safely tolerated. Furthermore, no negative effects of treatment were seen during the study nor in follow up 4 weeks after the completion of treatment. Longer term effects of exposure to Deep TMS treatment are not known. However, exposure to other devices (such as MRI scanners) with the same type and strength of magnetic fields produced by the BrainsWay Deep TMS coil are not associated with significant short-term or long-term safety concerns.
The safety and effectiveness of the BrainsWay Deep TMS System has not been established in the following patient populations or clinical conditions through a controlled clinical trial.
Table 1 below presents the adverse events reported in the clinical study in ≥5% or more of the patients who received the BrainsWay Deep TMS treatment or the sham (placebo) treatment. Safety information is provided from all patients who were treated in the clinical study.
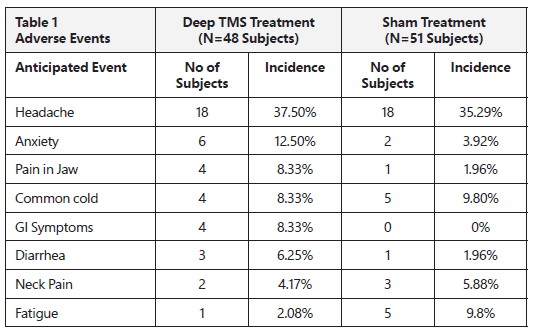
Headaches were reported in 37.5% of patients who received the BrainsWay Deep TMS treatment, and in 35.3% of the patients who received the sham (placebo) treatment. This indicates that headaches were not necessarily caused by the BrainsWay Deep TMS treatment. Headaches usually get better or go away altogether with successive treatments. Headaches may also be relieved by using common over-the-counter pain medications such as acetaminophen.
There were no notable differences in any of the reported adverse events in patients who received the BrainsWay Deep TMS treatment compared to patients who received the sham treatment. This indicates that these side effects were not necessarily caused by the BrainsWay Deep TMS treatment.
You should Inform the treatment administrator if you feel pain or discomfort during the treatment. The Deep TMS helmet may be slightly adjusted on your head to relieve the pain or discomfort. Pain and discomfort associated with treatment usually gets better or goes away altogether with successive treatments.
Other side effects which may occur but are not necessarily caused by the BrainsWay Deep TMS treatment include anxiety, pain in jaw, neck pain or fatigue.
The safety and effectiveness of the BrainsWay Deep TMS System for treatment of Obsessive-Compulsive Disorder was demonstrated in a prospective, double blind, randomized, controlled, multicenter trial. The study was conducted at 11 study sites in the United States (9 sites), Israel (1 site) and Canada (1 site). During the treatment phase, TMS sessions were performed daily for 6 weeks.
The primary endpoint was the change from baseline (i.e., the starting score before treatment) in a standard scale for measuring OCD symptoms, known as the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). The model estimated mean change from baseline in Y-BOCS scores in the Deep TMS group across 6 weeks compared to the sham group was statistically significant (p=0.0127). The response rate (meaning the percent of patients who had a reduction in Y-BOCS scores of at least 30%) was statistically significantly better in the Deep TMS group compared to the sham group. There was a statistically significant improvement in Clinical Global Impression – Improvement (CGI-I) and Clinical Global Impression – Severity (CGI-S) in patients treated with the Deep TMS treatment compared to the sham group.
The efficacy of the Deep TMS treatment was still seen at 10 weeks, based on a statistically significant change from baseline in Y-BOCS scores (p=0.0380) and a significantly better response rate in the Deep TMS group compared to the sham group (p=0.0057). No significant differences were found in the CGI-S and CGI-I scores at 10 weeks. However, the scores were better in the Deep TMS group than in the sham group.
You may ask your healthcare provider for more information regarding the clinical study.
The BrainsWay Deep TMS System has been shown to be safe and effective for the treatment of Obsessive-Compulsive Disorder (OCD). You should discuss the treatment with your healthcare provider to ensure that BrainsWay Deep TMS treatment is the right treatment for you.
Most patients who benefit from BrainsWay Deep TMS experience results by the second week of treatment. Some patients may experience results in less time. BrainsWay Deep TMS treatment should be administered daily for six weeks. This is the treatment schedule that has been demonstrated as safe and effective in the clinical study.
As with any OCD treatment, there is a risk that your OCD symptoms might worsen during BrainsWay Deep TMS treatment. Inform your healthcare provider immediately if your OCD symptoms worsen.
BrainsWay Deep TMS treatment has not been evaluated to be safe and effective for patients with suicidal ideation or who have recently attempted suicide.